ISSN 2764-1449 | ISSN (Online) 2764-1430


Filipe André Gonzalez1,2,3; João Pedro Silva4; Pedro Gaspar Costa5; Luís Bento5,6; Doroteia Silva7; Igor Milet8; Alexandre Pinto9; Miguel Tavares9
Abstract
INTRODUCTION: The current hemodynamic management in intensive care units (ICU) in Portugal has not been assessed. We designed an electronic survey to better understand practice and alignment with the most recent guidelines.
METHODS: A short questionnaire of 24 questions, addressing hemodynamic monitoring and management in the ICU, was shared on social networks or via email (e-blast®) by the authors and the Portuguese Society of Critical Care from May 11th to September 11th, 2023.
RESULTS: Globally, 174 valid questionnaires were available for analysis. 54% were intensive care specialists, and the rest were physicians in training. When approaching the patient in septic shock, the volume of fluid challenge differed between 500 mL (42%), 250 mL (30.5%), and 1000 mL (19%), and the most used type of fluids were balanced solutions (85.5% vs 14.4% normal saline). Norepinephrine was universally the first choice for vasopressor in septic shock and most responders considered adding steroids (96%) and vasopressin (in 66.1%). The most frequently used tool to assess fluid responsiveness and hemodynamic monitoring was echocardiography (97.1%), but 31% of responders considered that less than half of the medical staff was skilled in echocardiographic assessment.
CONCLUSION: Our survey suggests that most patients in septic shock receive fluids, norepinephrine, vasopressin, and steroids, according to current international recommendations. Echocardiography is the preferential tool for hemodynamic assessment. Still, most responders considered that the team was not skilled enough to perform it, thus highlighting the importance of training and standardization in this technique in the ICU.
INTRODUCTION
Adequate hemodynamic assessment and management are cornerstones in the care for the management of critically ill patients 1,2. However, bedside hemodynamic monitoring faces many challenges. First, methods, devices, and parameters available for hemodynamic monitoring have evolved over the last 30 years, and this may be responsible for the significant heterogeneity in the types of techniques used by clinicians in various intensive care units (ICUs). Second, properly using these monitoring tools and interpreting the variables displayed is complex and requires a high level of knowledge and skill, resulting in heterogeneous interventions3,4. Third, advanced methods for hemodynamic monitoring, per se, have not been associated with improving patient survival 5,6 unless coupled with early and clinically relevant therapeutic strategies 2,7. Considerable heterogeneity in the availability and practice of hemodynamic monitoring exists at the bedside across clinicians, ICUs, and countries, although studies investigating this issue are scarce 3,8,9.
OBJECTIVES
This study aimed to investigate the Portuguese status and potential regional differences regarding shock assessment, namely focusing on hemodynamic management and monitoring practices, therapeutic options, and hemodynamic monitoring devices. Also, there are a few guidelines on circulatory compromise and hemodynamic management and monitoring, such as the Consensus on Circulatory Shock and Hemodynamic Monitoring: Task Force of the European Society of Intensive Care Medicine10 and the Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 202111. Therefore, the second objective of this survey was to better understand current practices and alignment with these recent guidelines.
METHODS
A short questionnaire (see Appendix 1) was developed and posted online (Google Forms survey). Intensivists, both specialists and in training, working in Portuguese ICUs and involved in the care of critically ill patients, were invited to answer 24 questions about hemodynamic monitoring and management in the ICU.
From May 11, 2023, the survey was shared on social networks or via email (e-blast) by the authors and the Portuguese Society of Intensive Care (SPCI, spci.pt) and the association of Intensive care Medicine residents (AIMINT, ai
Questionnaires not filled by an intensivist (certified or trainee) or with more than three unanswered questions were considered invalid. Responses were monitored daily, and the database was locked in analysis after four months (September 11, 2023). Data are presented as numbers or percentages. Multiple answers were allowed for several questions (see Appendix 1). Therefore, cumulative percentages presented in the text may sometimes exceed 100%.
RESULTS
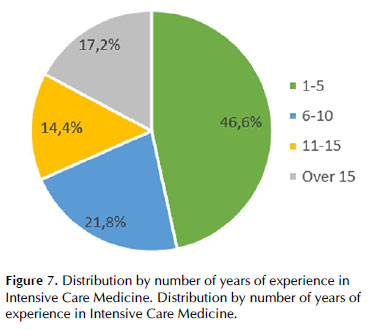
Globally, 174 valid questionnaires were available for analysis: 54% from intensive care specialists, 23.6% from intensive care residents, and 22.4% from other specialists in intensive care training. Regarding intensive care medicine practice experience, 46.6% had between 1 and 5 years, and 17.2% had more than 15 years (Figure 1). Most treated intensive care level III and II patients (94.3% and 86.8%, respectively), but only 14.3% treated level I patients.
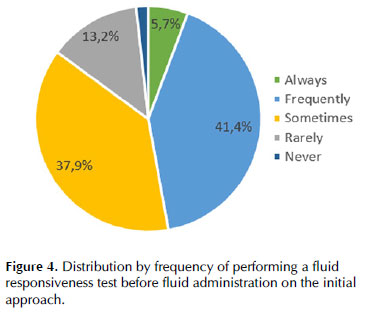
Regarding the initial approach of the patient in septic shock, the volume of fluid challenge and type of fluid used differed between clinicians, as depicted in Figures 2 and 3, respectively. Less than half of responders would perform a fluid responsiveness test before fluid administration on the initial approach (Figure 4).
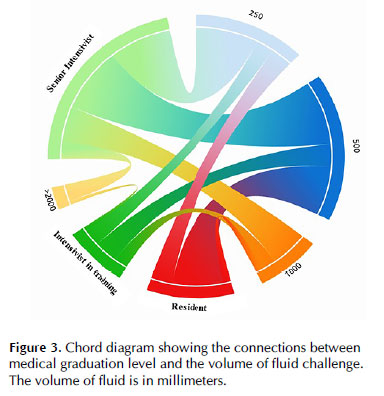
When correlating the volume of fluid challenge administered on the initial approach with the medical graduation level, there was a significant association between the more differentiated intensive care specialist (senior intensivist) and less volume administered in the fluid challenge (p = 0.01) when compared to intensivists in training and residents of intensive care medicine, as shown on Diagram 1.
The most common criteria to start vasopressors was MAP ≤65 mmHg after 30 mL/kg of fluid loading and poor peripheral perfusion (77.6% both), with norepinephrine being the universal first choice. For the second vasopressor, 66.1% considered adding vasopressin, and 45.4% considered adding epinephrine. Regarding adjuvant therapies for septic shock, we highlight the choice of steroids (96%) and albumin (63.8%). Radial insertion was the commonest site for invasive blood pressure continuous monitoring (75.3%). Most respondents (71.8%) considered decreasing the patient's sedation to improve hemodynamic status if the patient was sedated with a RASS -5.
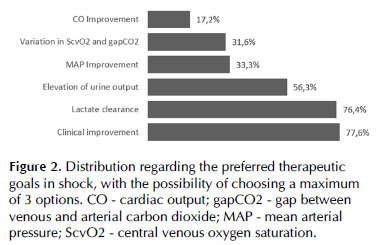
The most used therapeutic targets in shock were clinical improvement (77.6%) and lactate clearance (76.4%), according to Figure 5 (clinical improvement was defined as improvement in one or more organ hypoperfusion signs on the physical examination).
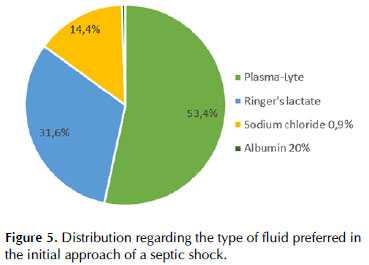
Volume status was evaluated routinely in 41.4% and sporadically in 37.9% of the cases. The most common assessment strategies were echocardiography-derived cardiac output combined with lung ultrasound evaluation (92.5%), physical examination (55.2%), and thermodilution techniques derived cardiac output and volume parameters (50.6%). Less frequently, clinicians used cardiac output measured by pulse contour analysis, bioreactance, PPV, CVP, or urinary output. The most frequently used tools to assess fluid responsiveness varied among responders but predominated the dynamic evaluation of IVC (52.3%), cardiac output measured by echocardiography (45.4%), and PPV calculation (44.8%).
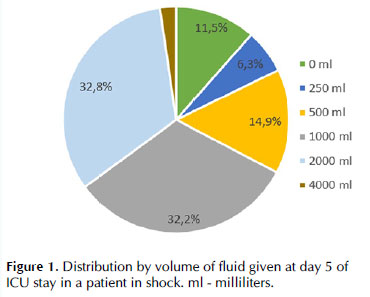
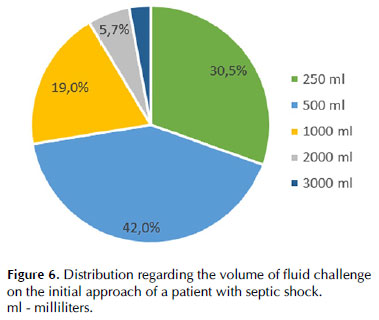
According to the responders, most patients received between 1000 and 2000 mL/day after five days in the ICU (Figure 6).
When questioned about how many patients received an echocardiographic evaluation at least once during their ICU stay, 60.3% of responders considered between 75-100%, 23% between 50-75%, and 9.8% between 25-50% of the patients. Regarding echocardiography competency to assess patients, 57.5% considered that more than half of the medical staff was skilled, 31% considered less than half of the medical staff, and 7.5% considered all the medical staff could do it (Figure 7).
When inquired about implementing measures to improve the utilization of hemodynamic monitoring tools and devices, more than half of the responders valued education, algorithm implementation, and participation in hemodynamic projects. Overall, there were no regional differences between hemodynamic management and monitoring practices.
DISCUSSION
The main findings of our survey could be summarized as follows: 1) most septic shock patients in Portuguese ICUs receive fluids, norepinephrine, vasopressin, and steroids; 2) echocardiography was the primary tool to assess cardiac output, volume status, fluid responsiveness, and hemodynamic monitoring; 3) almost a third of responders considered that more than half of the team was not skilled enough to perform echocardiography.
Initial management of patients with hemodynamic instability in Portuguese ICUs included fluid administration. However, the volume chosen for the fluid challenge varied between 250 (30.5%), 500 (42%), and 1000 mL (19%). The current literature recommends 4 mL/kg of volume in 10-15 minutes for the fluid challenge (280 mL for a 70 kg adult) 12,13. We could speculate that this mismatch could be partly explained by confusion between fluid concepts, such as "fluid challenge" vs. "fluid bolus" vs. "fluid resuscitation". According to SSC 11, at least 30 mL/kg of fluid is suggested in the first 3 hours, which would be more in line with the 1000 mL option of the responders. It is important to remember that the fluid challenge technique is first of all a test of the cardiocirculatory system. It allows the clinician to test whether the patient has a preload reserve that can be used to increase the SV with further fluids. These further fluids can be given after a positive response to a fluid challenge, or they can be given in a controlled way by repeating the fluid challenge as long as there is a positive response 14. This is very different from fluid bolus, in which fluids are given without real-time monitoring of the response. The only ‘excess' fluid that may be given with the fluid challenge technique is the amount of fluid used when the patient fails to respond. Also, the volume of fluid challenge differed among the clinicians, with less volume being administered by the more graduated intensivists when compared to residents or non-intensivists. This highlights the importance of education programs in hemodynamic management, especially in shock and fluid challenges, in the intensive care department, and in specialty graduation programs.
Despite consistent recommendations on using fluid responsiveness tests before fluid administration by SSC 2021 guidelines 11 and the ESICM 2014 guidelines 10, less than half of the responders used it frequently, which is in accordance with a previous study 13. Nevertheless, when used, responders always used dynamic instead of static parameters, such as the evaluation of IVC variation (52.3%), cardiac output variation measured by echocardiography (45.4%), and PPV calculation (44.8%). The prediction of fluid responsiveness with TTE requires the evaluation of the inferior vena cava (IVC) respiratory variations 15 or of the velocity time integral (VTI) respiratory variations recorded at the level of the left ventricular outflow tract 16. It is worth noticing that these variables have limited sensitivity in patients ventilated with a low tidal volume for protective mechanical ventilation. Indeed, in this context, if large IVC or VTI respiratory variations are highly suggestive of fluid responsiveness, small variations cannot exclude it (false negative). The same limitation applies to pulse pressure variation (PPV) and stroke volume variation (SVV), which were also popular methods among our respondents17. The passive leg raising maneuver (PLR) 18 and the end-expiratory occlusion test (EEOt) 19 are interesting alternatives to TTE-derived variables, PPV and SVV during protective mechanical ventilation 20 were less often used (PLR 38.5% and EEOt 21.8%).
The fluid challenge test 14, PLR test, and EEOt usually require the simultaneous use of a fast response cardiac output monitoring system (typically a pulse contour technique) to capture transient changes in stroke volume or cardiac output during the maneuver 18. Of note, although in clinical practice, an increase in mean arterial pressure following the elevation of a patient's legs is often used as a "passive leg raising test," a passive leg raising test in a narrow sense should follow a strict protocol. It should include the continuous assessment of CO 18. Also, it has been demonstrated that dynamic cardiac preload parameters (pulse pressure variation, stroke volume variation) cannot be used in a relevant proportion of critically ill ICU patients because mandatory criteria for their use (i.e., controlled ventilation, sinus rhythm) are not fulfilled 21. These limitations and technical aspects could account for the low compliance with fluid responsiveness tests before fluid administration.
Most respondents (71.8%) considered decreasing the patient's sedation to improve hemodynamic status if the patient was sedated with a RASS -5, which may reflect the arousal of the vasoplegic effects of most of the sedatives used in septic shock patients. This has to be counterbalanced by the benefits of decreasing overall metabolic demands on an already mismatched delivery-consumption state, a characteristic of sepsis. For critically ill patients, as with any other medical procedure they undergo in the ICU, such as hemodynamic monitoring or ventilation, the personalization of sedoanalgesia is the only way to obtain the best patient outcomes. Indeed, we must go beyond simply avoiding the use of certain group of drugs towards the concept of "objective-guided sedation", taking full advantage of the therapeutic arsenal currently available to us, as recently reviewed by Marcos-Vidal et al.22.
A hemodynamic monitoring device was considered in 59.2% for cardiac output measurement, mainly in the context of refractory shock (79.9%), undifferentiated shock (57.7%), or inconclusive echocardiographic evaluation (54.6%), which matches the ESICM 2014 10 and SSC 2021 guidelines (11) recommendations. The most used devices by the responders were echocardiography (97.1%), transpulmonary thermodilution system (82.2%), and pulmonary artery catheter (45.4%), like previous surveys done in Brazil, Germany, Switzerland, and worldwide 23–27.
Our survey shows a clear predominance in the use of echocardiography as a diagnostic and monitoring tool. Still, when questioned about how many patients received an echocardiographic evaluation at least once during their ICU stay, only 60.3% of responders considered between 75-100%, and 31% considered that less than half of the medical staff was skilled for an echocardiographic assessment. This perspective of the responders can be biased, first, by the prevalence of 46% of intensivists in training and less experienced (between 1 and 5 years), second, by the lack of specification on the level of training in echocardiography in this question. This gap in echocardiography experience and training could be improved by a national educational program including more ultrasound courses stratified by levels of expertise, more training time in the Cardiology echocardiography laboratory, more comprehensive and frequent simulator training, implementation of echocardiography internships in the ICU environment (with certificated mentors, such as European Diploma of Advanced Critical Care Echocardiography – EDEC – or European Association of Cardiovascular Imaging (EACVI) Certification in Adult Transthoracic Echocardiography (TTE)).
Recently, the ESICM released an expert-based consensus regarding the basic skills for head‑to‑toe ultrasonography in the intensive care setting 28, where it recommends that the evaluation of LV outflow tract velocity time integral as an estimation of stroke volume should be a basic skill. Also, some previous studies have demonstrated that critical care echocardiography performed at the bedside by intensivists with basic critical care echocardiography training is an accurate and reproducible technique to measure cardiac output in critically ill patients29,30. Nevertheless, there is growing evidence demonstrating that machine learning automatic tools in ultrasound machines can accurately help inexperienced clinicians acquire essential measurements for hemodynamic management, such as left ventricle outflow tract VTI 31 and left ventricle ejection fraction 32,33.
The traditional approach to the shocked patient has been administering fluids until the patient is no longer fluid-responsive. However, this strategy may lead to fluid overload 20. Although fluid overload is associated with increased morbidity and mortality, no clear parameters guide the physician on when to stop fluid administration. Clinical and imaging variables suggest the presence of interstitial edema occurs late. In 1984, Shippy 34 analyzed fluid therapy and its relationship to variables such as central venous pressure (CVP), concluding that they do not adequately reflect the volume status of critically ill patients. Therefore, they are not currently recommended for guiding fluid removal 35,36. It has been observed that the normal maximum diameter of the IVC ranges from 1.9 to 2.1 cm; patients presenting with an IVC diameter close to this, with minimal or no variation during the respiratory cycle, do not benefit from IV fluids37. The use of the IVC collapsibility index does have some limitations, including inter-observer differences, high rates of false positives, and mild-to-moderate positive predictive value, as discussed in the review paper by Via et al. 38. The use of VExUS relies on the measurement of the inferior vena cava's size in conjunction with Doppler flow interrogation at the level of the hepatic, portal, and intra-renal veins. It may be useful in assessing systemic venous congestion and directing fluid management 39, helping clinical decision-making regarding fluid tolerance. Also, LUS has been confirmed to be a rapid, non-invasive, and reproducible bedside tool to estimate the extra-vascular lung water 40, and it is turning into a key component for determining the presence of pulmonary edema and estimating its severity in different clinical contexts 41,42; hence, it could help to understand whether there is a clinically relevant impact of increased LA pressure.
Our study has limitations. In addition to emails that are clearly targeted, we used social networks (LinkedIn, Twitter, WhatsApp) to invite clinicians to answer the survey and share the link. Therefore, we could not control the number of clinicians who received the survey and hence determine the percentage of respondents. Furthermore, we can have a bias phenomenon in which clinicians who are more interested and skilled in the subject are more motivated to answer. However, regarding the Portuguese population and the ratio of intensivists, our survey had fewer participants than previous surveys in other bigger countries 23–26. All the questions on this survey had close-ended answers, which conditions the respondents' options and perspectives and sometimes could lead to misinterpretations. Finally, this is a survey, not an audit or an observational study. Therefore, the clinician's feedback reflects their perception of what is done in their unit, which may sometimes differ from reality.
CONCLUSION
Resuscitation practices in Portuguese ICUs regarding fluid administration, norepinephrine, and steroid usage are following current international recommendations. Echocardiography is a preferential tool for hemodynamic monitoring, assessing volume status and fluid responsiveness. Nevertheless, according to about a third of the surveyed physicians, there are gaps in skill and qualifications to adequately perform advanced echocardiography in more than half of the medical team. These results highlight the importance of training and standardization in this technique.
DECLARATION OF AUTHORS' CONTRIBUTION
All authors contributed equally from the project design, data collection and analysis, to the writing and reviewing.
REFERENCES
1. Lees N, Hamilton M, Rhodes A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit Care. 2009;13(5). doi:10.1186/CC8039
2. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377. doi:10.1056/NEJMOA010307
3. Gnaegi A, Feihl F, Perret C. Intensive care physicians' insufficient knowledge of right- heart catheterization at the bedside: time to act? Crit Care Med. 1997;25(2):213-220. doi:10.1097/00003246-199702000-00003
4. Jain M, Canham M, Upadhyay D, et al. Variability in interventions with pulmonary artery catheter data. Intensive Care Med. 2003;29(11):2059-2062. doi:10.1007/S00134- 003-1924-7
5. Harvey S, Stevens K, Harrison D, et al. An evaluation of the clinical and cost- effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial. Health Technol Assess. 2006;10(29). doi:10.3310/HTA10290
6. Connors AF, Speroff T, Dawson N V., et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276(11):889-897. doi:10.1001/JAMA.276.11.889
7. HP W, AP W, GR B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575. doi:10.1056/NEJMOA062200
8. Torgersen C, Dünser MW, Schmittinger CA, et al. Current approach to the haemodynamic management of septic shock patients in European intensive care units: a cross-sectional, self-reported questionnaire-based survey. Eur J Anaesthesiol. 2011;28(4):284-290. doi:10.1097/EJA.0B013E3283405062
9. Kastrup M, Markewitz A, Spies C, et al. Current practice of hemodynamic monitoring and vasopressor and inotropic therapy in post-operative cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand. 2007;51(3):347-358. doi:10.1111/J.1399-6576.2006.01190.X
10. Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795-1815. doi:10.1007/S00134-014-3525- Z
11. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi:10.1007/S00134-021-06506-Y
12. Aya HD, Rhodes A, Chis Ster I, et al. Hemodynamic Effect of Different Doses of Fluids for a Fluid Challenge: A Quasi-Randomized Controlled Study. Crit Care Med. 2017;45(2):e161-e168. doi:10.1097/CCM.0000000000002067
13. Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study. Intensive Care Med. 2015;41(9):1529-1537. doi:10.1007/s00134-015-3850-x
14. Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17(3):290-295. doi:10.1097/MCC.0b013e32834699cd
15. Feissel M, Michard F, Faller JP, et al. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9). doi:10.1007/s00134-004-2233-5
16. Feissel M, Michard F, Mangin I, et al. Respiratory Changes in Aortic Blood Velocity as an Indicator of Fluid Responsiveness in Ventilated Patients With Septic Shock. Chest. 2001;119(3):867-873. doi:10.1378/chest.119.3.867
17. Michard F, Chemla D, Teboul JL. Applicability of pulse pressure variation: how many shades of grey? Crit Care. 2015;19(1):144. doi:10.1186/s13054-015-0869-x
18. Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care. 2015;19(1):18. doi:10.1186/s13054-014-0708-5
19. Gavelli F, Teboul JL, Monnet X. The end-expiratory occlusion test: please, let me hold your breath! Crit Care. 2019;23(1):274. doi:10.1186/s13054-019-2554-y
20. Malbrain MLNG, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66. doi:10.1186/s13613-018-0402-x
21. Mair S, Tschirdewahn J, Götz S, et al. Applicability of stroke volume variation in patients of a general intensive care unit: a longitudinal observational study. J Clin Monit Comput. 2017;31(6):1177-1187. doi:10.1007/s10877-016-9951-4
22. Marcos-Vidal JM, González R, Merino M, et al. Sedation for Patients with Sepsis: Towards a Personalised Approach. Journal of Personalized Medicine 2023, Vol 13, Page 1641. 2023;13(12):1641. doi:10.3390/JPM13121641
23. Funcke S, Sander M, Goepfert MS, et al. Practice of hemodynamic monitoring and management in German, Austrian, and Swiss intensive care units: the multicenter cross- sectional ICU-CardioMan Study. Ann Intensive Care. 2016;6(1):49. doi:10.1186/s13613-016-0148-2
24. Saugel B, Reese PC, Wagner JY, et al. Advanced hemodynamic monitoring in intensive care medicine. Med Klin Intensivmed Notfmed. 2018;113(3):192-201. doi:10.1007/s00063-017-0302-0
25. Dias FS, Rezende EA de C, Mendes CL, et al. Hemodynamic monitoring in the intensive care unit: a Brazilian perspective. Rev Bras Ter Intensiva. 2014;26(4). doi:10.5935/0103- 507X.20140055
26. Siegenthaler N, Giraud R, Saxer T, et al. Haemodynamic monitoring in the intensive care unit: results from a web-based Swiss survey. Biomed Res Int. 2014;2014. doi:10.1155/2014/129593
27. Michard F, Malbrain ML, Martin GS, et al. Haemodynamic monitoring and management in COVID-19 intensive care patients: an International survey. Anaesth Crit Care Pain Med. 2020;39(5):563-569. doi:10.1016/j.accpm.2020.08.001
28. Robba C, Wong A, Poole D, et al. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: consensus and expert recommendations of the European Society of Intensive Care Medicine . Intensive Care Med. 2021;47(12):1347-1367. doi:10.1007/s00134-021-06486-z
29. Vignon P, Begot E, Mari A, et al. Hemodynamic Assessment of Patients With Septic Shock Using Transpulmonary Thermodilution and Critical Care Echocardiography. Chest. 2018;153(1):55-64. doi:10.1016/j.chest.2017.08.022
30. Villavicencio C, Leache J, Marin J, et al. Basic critical care echocardiography training of intensivists allows reproducible and reliable measurements of cardiac output. Ultrasound J. 2019;11(1):5. doi:10.1186/s13089-019-0120-0
31. Gonzalez FA, Varudo R, Leote J, et al. Automation of sub-aortic velocity time integral measurements by transthoracic echocardiography: clinical evaluation of an artificial intelligence-enabled tool in critically ill patients. Br J Anaesth. 2022;129(5):e116-e119. doi:10.1016/j.bja.2022.07.037
32. Varudo R, Gonzalez FA, Leote J, et al. Machine learning for the real-time assessment of left ventricular ejection fraction in critically ill patients: a bedside evaluation by novices and experts in echocardiography. Crit Care. 2022;26(1):386. doi:10.1186/s13054-022- 04269-6
33. Bacariza J, Gonzalez FA, Varudo R, et al. Smartphone-based automatic assessment of left ventricular ejection fraction with a silicon chip ultrasound probe: a prospective comparison study in critically ill patients. Br J Anaesth. 2023;130(6):e485-e487. doi:10.1016/j.bja.2023.02.032
34. Shippy CR, Appel PL, Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med. 1984;12(2):107-112. doi:10.1097/00003246-198402000-00005
35. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178. doi:10.1378/CHEST.07-2331
36. Magder S. More respect for the CVP. Intensive Care Med. 1998;24(7):651-653. doi:10.1007/S001340050640
37. Orso D, Paoli I, Piani T, et al. Accuracy of Ultrasonographic Measurements of Inferior Vena Cava to Determine Fluid Responsiveness: A Systematic Review and Meta- Analysis. J Intensive Care Med. 2020;35(4):354-363. doi:10.1177/0885066617752308
38. Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-1167. doi:10.1007/S00134-016-4357-9
39. Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound Journal. Published online 2020. doi:10.1186/s13089-020-00163-w
40. Enghard P, Rademacher S, Nee J, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19(1):36. doi:10.1186/s13054-015-0756-5
41. Ferré A, Guillot M, Lichtenstein D, et al. Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Med. 2019;45(5):601-608. doi:10.1007/s00134-019-05573-6
42. Yang F, Wang Q, Zhang L, et al. Prognostic value of pulmonary oedema assessed by lung ultrasound in patient with acute heart failure. Heart Vessels. 2021;36(4):518-527. doi:10.1007/s00380-020-01719-5