ISSN 2764-1449 | ISSN (Online) 2764-1430


Henry Martins Soares Fortes1; Pedro Pinheiro Barros1; Letícia Lima Freitas1; Yne Kivia Dikauá Santos Feitosa1; Lorenna Rodrigues Pellegrino de Azevedo1; Rodrigo Simões Duarte Severiano1; Pedro Sá de Oliveira Costa1; Maria Letícia de Melo Santana1; Fernando José Pinho Queiroga Júnior1,2
Abstract
This study proposes to review the available scientific literature on EVALI, to discuss its epidemiology, clinical features, diagnosis, and treatment of the disease. For this, the PubMed database was accessed, using the following descriptors: “EVALI”, “E-Cigarette”, “Acute lung injury”, “Respiratory failure”, “Vaping” and “Juuling”. Epidemiologically, the disease is more frequent in young male adults, and high rates of associated psychiatric illnesses have been observed in studies in this population. Clinically, EVALI presents non-specifically, mainly with respiratory and gastrointestinal symptoms, being diagnosed with a history of vaping in the last 90 days, consistent radiological findings and exclusion of other diagnoses. Treatment is similar to other forms of respiratory failure, with oxygen therapy and ventilatory support if saturation <95, in addition to early treatment with corticosteroids and antimicrobial therapy. Due to the non-standardization of electronic cigarettes and the recent identification of the disease, further studies are still needed to establish more specific guidelines for its diagnosis and treatment.
INTRODUCTION
Since 2014, a new tobacco product, electronic cigarettes, has been widely used by the young population, especially teenagers, which has brought great concern to pulmonologists[1,2]. This new product consists of an electronic device designed to vaporize chemical compounds, whether or not they contain nicotine[3]. Depending on the model, the use of these devices is called vaping or Juuling[4].
Late effects still remain unknown, due both to the lack of manufacturing standards, making it difficult to predict pulmonary exposure for the generic class of e-cigarettes, and to the relatively recent introduction of the device to the market[5]. However, even though they are sold as a safer alternative to conventional tobacco products or as a transition for individuals who wish to quit smoking[6,17], the early consequences of the use of these devices have been observed and there are already reports of fatal cases. of acute respiratory failure triggered by these devices[8-10].
The first report of significant lung disease from vaping was in 2019 in the United States[11]. The term EVALI (E-cigarette or Vaping product use-Associated Lung Injury) was introduced shortly afterwards, simultaneously with the increase in the number of cases of the disease[12]. In this sense, the present study aims to review the available scientific literature on EVALI.
METHODOLOGY
This is a descriptive and exploratory study, a narrative review of the literature, including studies that address EVALI. For this, the PubMed database was accessed, using the following descriptors: “EVALI”, “E-Cigarette”, “Acute lung injury”, “Respiratory failure”, “Vaping” and “Juuling”. We also included relevant studies found in the reference list of selected studies.
EPIDEMIOLOGY
Patients with EVALI are mostly young adult males[13]. In 2020, a multicenter study analyzed 2558 non-fatal and 50 fatal cases of the disease, noting a predominance of men in both categories (67% and 53%, respectively). Age, on the other hand, showed divergence between the categories: 78% of non-fatal cases were in individuals under 35 years of age, while 73% of fatal cases were in individuals over 35 years of age. In addition, both groups had high levels of psychiatric disorders (41% and 65%), indicating a possible risk factor for e-cigarette use[14].
PATHOPHYSIOLOGY AND CLINICAL PRESENTATION
As mentioned above, as there is no standardization of substances present in these devices, there is no absolute mechanism for all of them. However, devices with nicotine have mostly propylene glycol (PG) and glycerol (GLY), substances known for their irritating characteristic of the respiratory tract, leading to obstruction and inflammation[15-17]. In addition, PG and GLY do not cross biological membranes and may induce a state of hyperosmotic stress, leading to the secretion of pro-inflammatory cytokines in the lung[18-20]. This mechanism can activate inflammatory cascades that result in surfactant disturbance and small airway collapse, modifying ventilation/perfusion regulation and thus interfering with gas exchange[21]. Furthermore, nicotine, present in more than 90% of e-cigarettes, can cause bronchoconstriction of the large airways when inhaled[22].
Few patients diagnosed with EVALI underwent lung biopsy, but the histopathological findings are consistent with acute lung injury such as acute fibrosing pneumonia, diffuse alveolar damage, and foamy macrophages[23]. In addition, CDC researchers have already identified Vitamin E acetate in the bronchoalveolar lavage of patients with EVALI as a possible factor of disease severity. The proposed mechanism is the disruption of alveolar surfactant, leading to a decrease in surface tension, alveolar collapse and lung injury[24].
Classic clinical findings consist of nonspecific respiratory symptoms such as shortness of breath, chest pain, cough and/or hemoptysis. Most patients also report gastrointestinal symptoms such as nausea, vomiting and/or diarrhea and constitutional symptoms such as fever, chills, weight loss and fatigue[25]. Werner et al. [14] observed a lower incidence of gastrointestinal symptoms in fatal cases than in non-fatal cases (53% and 80% respectively), indicating a greater severity of the disease in the predominantly respiratory form. Laboratory findings are also nonspecific, with an elevation of leukocyte count and erythrocyte sedimentation rate being present[25].
DIAGNOSTIC AND IMAGING EXAMS
According to the CDC, the diagnostic criteria for EVALI are: history of vaping within the last 90 days, consistent radiological findings, and ruling out infection and other differential diagnoses. However, some pulmonologists include bronchoscopy, bronchoalveolar lavage, and lung biopsy[26].
In general, EVALI presents nonspecific findings on imaging tests. The most frequently reported findings are bilateral ground-glass opacities with areas of consolidation, often with a peculiar pattern of subpleural sparing and the presence of a reverse halo sign[25] (Figure 1).
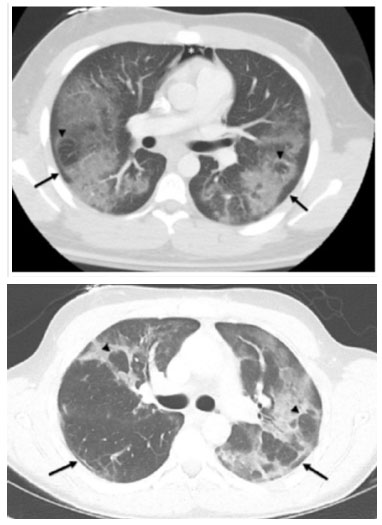
Imaging findings usually bring a differential diagnosis that includes infection and a variety of patterns of acute lung injury, including pneumonia and diffuse alveolar damage. The main imaging findings and the corresponding lung injury are summarized in (Table 1).
In a multicentric cohort, Kligerman et al. [28] analyzed chest tomography (CT) scans of 160 patients with EVALI. In this study, most patients had a predominantly ground-glass symmetric lung lesion, with the level of consolidation proportional to the severity of the lesion (p<0.033). According to the imaging findings, ninety-seven and a half percent (97.5%) of the patients were classified into the following patterns: diffuse alveolar damage, acute eosinophilic pneumonia, diffuse alveolar hemorrhage, diffuse parenchymal pneumonia, upper lobe pneumonia, and mixed pneumonia.
In addition, a significant difference was observed in the presence of peribronchovascular preservation in relation to the qualitative severity of lung injury on CT (p < 0.007), with less preservation in those with a milder form of the disease. Compared with those without, patients with preservation were significantly younger (p < 0.016). Other patterns observed were interlobular septa thickening (50.6%) and crazy-paving pattern (18.8%). Interlobular septa thickening, pleural effusion and lymphadenopathy were also associated with lesion severity (p<0.013, p=0.016 and p=0.007, respectively).
TREATMENT
According to CDC recommendations, patients with suspected EVALI and saturation <95% in room air should be hospitalized, with oxygen therapy and ventilation support, such as invasive mechanical ventilation, in a protective strategy, similar to that used in acute respiratory distress syndrome (ARDS)[29].
As with other causes of respiratory insufficiency, EVALI is treated early with corticosteroids and antimicrobial therapy[12]. Corticosteroids were the treatment of choice in most case series, with satisfactory results[30]. There are still no recommendations for doses and duration of treatment, with discharge only if clinical stability is guaranteed[29].
CONCLUSIONS
EVALI is a recent pathology with a nonspecific clinical presentation, being diagnosed mainly through a recent history of vaping and radiological exams with consistent findings.
Therefore, with the increasing use of electronic cigarettes, EVALI becomes an important etiology to be considered in cases of respiratory failure, especially among young people. However, the scientific literature is still limited by the non-standardization of these devices, making it impossible to identify all the substances involved in the pathophysiology of the disease.
REFERENCES
1. Bizoń M, Maciejewski D, Kolonko J. E-cigarette or vaping product use-associated acute lung injury (EVALI) as a therapeutic problem in anaesthesiology and intensive care departments. Anaesthesiol Intensive Ther. 2020;52(3):219-225. doi: 10.5114/ait.2020.97989. PMID: 32876409.
2. Oriakhi M. Vaping: An Emerging Health Hazard. Cureus. 2020 Mar 26;12(3):e7421. doi: 10.7759/cureus.7421. PMID: 32351806; PMCID: PMC7186084.
3. Lampos S, Kostenidou E, Farsalinos K, Zagoriti Z, Ntoukas A, Dalamarinis K, Savranakis P, Lagoumintzis G, Poulas K. Real-Time Assessment of E-Cigarettes and Conventional Cigarettes Emissions: Aerosol Size Distributions, Mass and Number Concentrations. Toxics. 2019; 7(3):45. https://doi.org/10.3390/toxics7030045
4. El Dib R, Suzumura EA, Akl EA, et al. Electronic nicotine delivery systems and/or electronic non-nicotine delivery systems for tobacco smoking cessation or reduction: a systematic review and meta-analy-sis. BMJ Open 2017; 7: e012680. doi: 10.1136/bmjopen-2016-012680
5. Cobb NK, Solanki JN. E-Cigarettes, Vaping Devices, and Acute Lung Injury. Respir Care. 2020 May;65(5):713-718. doi: 10.4187/respcare.07733. PMID: 32345762.
6. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of aneLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study.PLoS One. 2013;8(6):e66317.
7. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis.Lancet Respir Med.2016;4(2):116–128.
8. Perrine CG, Pickens CM, Boehmer TK, et al. Characteristics of a multistate outbreak of lung injury associated with e-cigarette use, or vaping – United States, 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 860-864. doi: 10.15585/mmwr.mm6839e1.
9. Lewis N, McCaffrey K, Sage K, et al. E-cigarette Use, or Vaping, Practices and Characteristics Among Persons with Associated Lung Injury – Utah, April-October 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 953-956. doi: 10.15585/mmwr.mm6842e1.
10. Hajek P, Phillips-Waller A, Przulj D, et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol Assess 2019; 23: 1-82
11. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use - interim guidance.MMWR Morb MortalWkly Rep. 2019;68(36):787–790.
12. Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury - United States, October2019.MMWR Morb Mortal Wkly Rep. 2019;68(41):919–927.
13. Krishnasamy VP, Hallowell BD, Ko JY, Board A, Hartnett KP,Salvatore PP, et al; Lung Injury Response Epidemiology/SurveillanceTask Force. Update: characteristics of a nationwide outbreak of e-ciga-rette, or vaping, product use–associated lung injury— United States,August 2019–January 2020. MMWR Morb Mortal Wkly Rep 2020;69(3):90- 94
14. Werner AK, Koumans EH, Chatham-Stephens K, Salvatore PP, Armatas C, Byers P, Clark CR, Ghinai I, Holzbauer SM, Navarette KA, Danielson ML, Ellington S, Moritz ED, Petersen EE, Kiernan EA, Baldwin GT, Briss P, Jones CM, King BA, Krishnasamy V, Rose DA, Reagan-Steiner S; Lung Injury Response Mortality Working Group. Hospitalizations and Deaths Associated with EVALI. N Engl J Med. 2020 Apr 23;382(17):1589-1598. doi: 10.1056/NEJMoa1915314. PMID: 32320569; PMCID: PMC8826745.
15. Wieslander G, Norba ck D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 2001;58(10):649-655.
16. Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging 34: 171–177, 2014. doi: 10.1111/cpf.12085.
17. Palazzolo DL, Nelson JM, Ely EA, Crow AP, Distin J, Kunigelis SC. The effects of electronic cigarette (ECIG)-generated aerosol and conventional cigarette smoke on the mucociliary transport velocity (MTV) using the bullfrog (R. catesbiana) palate paradigm. Front Physiol 8: 1023, 2017. doi: 10.3389/fphys.2017.01023.
18. Fowles JR, Banton MI, Pottenger LH. A toxicological review of the propylene glycols. Crit Rev Toxicol 43: 363–390, 2013. doi: 10.3109/10408444.2013.792328.
19. Frank MS, Nahata MC, Hilty MD. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1: 147–160, 1981. doi: 10.1002/j.1875-9114.1981.tb03562.x.
20. Iskandar AR, Gonzalez-Suarez I, Majeed S, Marescotti D, Sewer A, Xiang Y, Leroy P, Guedj E, Mathis C, Schaller JP, Vanscheeuwijck P, Frentzel S, Martin F, Ivanov NV, Peitsch MC, Hoeng J. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol Mech Methods 26: 392–416, 2016. doi: 10.3109/15376516.2016.1170251.
21. Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, Starczewska E, Briki R, de Hemptinne Q, Zaher W, Debbas N. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019 May 1;316(5):L705- L719. doi: 10.1152/ajplung.00492.2018. Epub 2019 Feb 6. PMID: 30724099; PMCID: PMC6589591.
22. Lee LY, Lin RL, Khosravi M, Xu F. Reflex bronchoconstriction evoked by inhaled nicotine aerosol in guinea pigs: role of the nicotinic acetylcholine receptor. J Appl Physiol (1985) 125: 117–123, 2018. doi: 10.1152/japplphysiol.01039.2017.
23. Bhatt JM, Ramphul M, Bush A. An update on controversies in e-cigarettes. Paediatr Respir Rev. 2020 Nov;36:75-86. doi: 10.1016/j.prrv.2020.09.003. Epub 2020 Sep 26. PMID: 33071065; PMCID: PMC7518964.
24. Blount BC, Karwowski MP, Shields PG, MorelEspinosa M, Valentin-Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D, Corstvet J, Cowan E, De Jesús VR, Espinosa P, Fernandez C, Holder C, Kuklenyik Z, Kusovschi JD, Newman C, Reis GB, Rees J, Reese C, Silva L, Seyler T, Song MA, Sosnoff C, Spitzer CR, Tevis D, Wang L, Watson C, Wewers MD, Xia B, Heitkemper DT, Ghinai I, Layden J, Briss P, King BA, Delaney LJ, Jones CM, Baldwin GT, Patel A, Meaney-Delman D, Rose D, Krishnasamy V, Barr JR, Thomas J, Pirkle JL; Lung Injury Response Laboratory Working Group: Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med 2020; 382:697–705
25. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - preliminary report.N Engl J Med. 2020;382(10):903–916.
26. Cecchini MJ, Mukhopadhyay S, Arrossi AV, Beasley MB, Butt YM, Jones KD, Pambuccian S, Mehrad M, Monaco SE, Saqi A, Smith ML, Tazelaar HD, Larsen BT. E-Cigarette or Vaping Product Use-Associated Lung Injury: A Review for Pathologists. Arch Pathol Lab Med. 2020 Dec 1;144(12):1490-1500. doi: 10.5858/arpa.2020-0024-RA. PMID: 32401055.
27. Chidambaram AG, Dennis RA, Biko DM, Hook M, Allen J, Rapp JB. Clinical and radiological characteristics of e-cigarette or vaping product use associated lung injury. Emerg Radiol. 2020 Oct;27(5):495-501. doi: 10.1007/s10140-020-01796-z. Epub 2020 May 28. PMID: 32462343; PMCID: PMC7906289.
28. Kligerman SJ, Kay FU, Raptis CA, Henry TS, Sechrist JW, Walker CM, Vargas D, Filev PD, Chung MS, Digumarthy SR, Ropp AM, Mohammed TL, Pope KW, Marquis KM, Chung JH, Kanne JP. CT Findings and Patterns of e-Cigarette or Vaping Product Use- Associated Lung Injury: A Multicenter Cohort of 160 Cases. Chest. 2021 Oct;160(4):1492- 1511. doi: 10.1016/j.chest.2021.04.054. Epub 2021 May 3. PMID: 33957099; PMCID: PMC8546241.
29. Evans ME, Twentyman E, Click ES, Goodman AB, Weissman DN,Kiernan E, et al. ; Lung Injury Response Clinical Working Group.Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States. MMWR MorbMortal Wkly Rep 2020;68(5152):1189-1194.
30. Jatlaoui TC, Wiltz JL, Kabbani S, Siegel DA, Koppaka R, MontandonM, et al; Lung Injury Response Clinical Working Group. Update: in-terim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury -United States, November 2019. MMWR Morb Mortal Wkly Rep2019;68(46):1081- 1086.